LSD variation should serve to treat schizophrenia-diepresse.com
Tailor-made molecule: US chemists synthesized a connection that almost resembles the LSD. The small difference causes that it does not work hallucinogen, but can be used against psychiatric diseases.
« And, can you synthesize LSD now? » Long after the hippie era, joke bolds asked you this question if you had outed yourself as a chemistry student. The true but bland answer: the total synthesis of LSD – for example from the amino acid tryptophan – is much more complex and expensive than to have the ergot mushroom produced the lysergic acid naturally and then make the lysergicediamide (LSD) in one step.
Jeremy R. Tuck, chemist at the University of California in Davis, is still dedicated to the synthesis of the LSD, she has already perfected. With a ulterior motive: he and other researchers of the working group of his doctor, David Olson, want to create molecules that differ little from the LSD – with the hope of using them as active ingredients against psychiatric diseases such as schizophrenia, but without – or at least with less – hallucinogenic effect. Because, to put it bluntly: if you already suffer from schizophrenia, you cannot need confusion and hallucinations. However, the beneficial property of LSD to work against the loss of neuronal connections that occur in schizophrenia and psychoses and even promote the formation of new neurons. This is called neuroplasticity.
A nitrogen atom is now located elsewhere
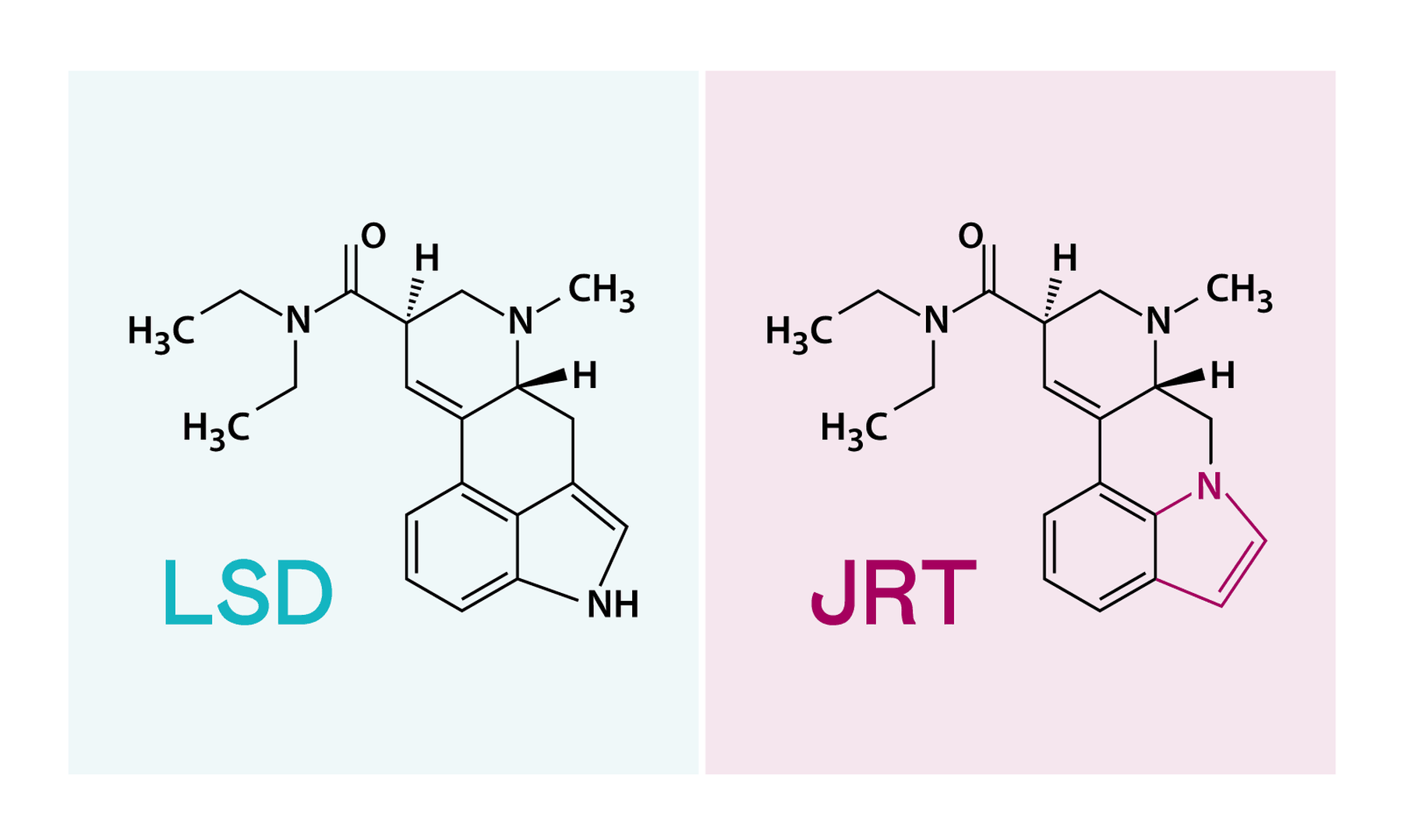
How does LSD work? Above all, by binding itself to a receptor in the brain that is actually intended for the neurotransmitter serotonin. Tuck and colleagues looked at the interaction of the LSD molecule and other neuroactive molecules with this serotonin receptor. Then they made the design of the desired connection, first on the paper: it is like the LSD with one difference: a nitrogen atom sits two places further in one of the four rings (see picture), and a hydrogen atom hangs on it. They called it JRT.
So the goal was clear, but not the way: JRT cannot be created in a simple way from lysergic acid or LSD, it does not make chemistry so easy. You have to rebuild the ring system, i.e. develop a total synthesis of this molecule from a commercially available substance. That took five years. Then the properties of JRT could be tested, partly in cell cultures, partly on rats. According to the report, the result is gratifying in « PNAs » (14.4): JRT only binds to receptors for serotonin, not for other neurotransmitters such as dopamine; It promotes neuroplasticity and cognitive performance; It does not create hallucinations (as far as you can show this by experiments on rats); It looks antidepressant (there is a standard test on rats!). In short, « it has extremely high therapeutic potential, » enthuses Olson. This does not mean that it can be prescribed patients, there are still many tests to follow.
In any case, the name of the fabric seems to be fixed. Where he comes from, some will ponder. The answer is simple: JRT are the initials of Jeremy R. Tuck. Chemists also like to immortalize themselves.








