Leuven Biotech company test new and improved Alzheimermedicijn
© AP
The Leuven biotech company Remynd is currently testing a new and improved Alzheimermedicijnlijd, which could help both patients in an early and advanced stage of the disease. The company itself announced this in a press release. The first results are expected at the beginning of July.
Source: Belga
Today at 07:36
The improved agent is based on the drug REM46127, with which Remynd conducted investigations last year. Participants who were then treated with the medicine clearly scored better in a memory test. Brain scans registered a better brain activity, and increased dopaminelevels were measured.
The levels of Tau proteins in the brain, which play a role in Alzheimer’s disease, were also significantly lower compared to the levels of the participants who received a placeboer. However, the study had to be stopped because side effects on the liver occurred in patients.
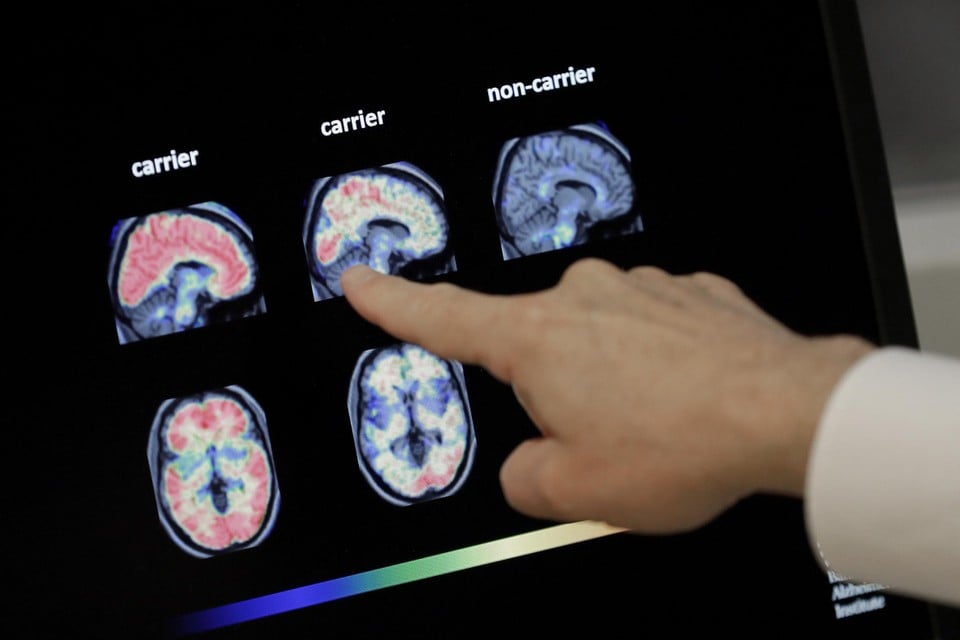
Read too. Researchers KU Leuven predict the start of Alzheimer’s with a genetic model
Now the company has a new molecule ready, which acts in the same way as the previous medicine, but that would no longer yield side effects. It also works on septine proteins, which help to control the calcium balance in brain cells. That balance was disturbed in Alzheimer’s disease.
Combination
« As with cancer, Alzheimer’s disease will probably be treated in the future with a combination of different medicines, » says Floor Stam, CEO of Remynd. “The mechanism of action of our candidate medicine is complementary to that of Leqembi (the drug that received green light from the European Commission in mid-April, ed.) And other means that are located in an advanced developmental stage. That could both be suitable as part of a combination therapy in the early stages of the disease. still empty -handed. ”
The new means will be tested by healthy volunteers in the coming month, to check safety and to better map their inclusion in the body. In the next phase, the intention is also to test it with Alzheimer patients.








:format(webp)/s3/static.nrc.nl/images/gn4/stripped/data130990529-68d285.jpg)